Why Do Atoms Form Chemical Bonds With Other Atoms - A helium atom (atomic number 2), has two protons and two electrons.
Why Do Atoms Form Chemical Bonds With Other Atoms - A covalent bond is formed when atoms share valence electrons. Bonds form when atoms share or transfer valence electrons. To understand why atoms form molecules. There are three different types of. Ad browse & discover thousands of science book titles, for less.
Web just as the structure of the atom is held together by the electrostatic attraction between the positively charged nucleus and the negatively charged electrons surrounding it, the stability within chemical bonds is also due to electrostatic attractions. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. Interpret the properties of elements that are important for life from the periodic table. Web a chemical bond is a force of attraction between atoms or ions. It becomes a positive hydrogen ion (h+). The cf bond in cf, (chang, 11 edition, 9.39) 3. Why do some elements exist as molecules in nature instead of as free atoms?
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Atoms form two basic bonds, covalent or ionic bonds, to fill the full outer shell of electrons. Atomic structure of carbon atom showing the particles of an atom: Web all models of chemical bonding have three common features: An ionic bond is formed when one atom accepts or donates one or more of its valence.
Covalent bond Definition, Properties, Examples, & Facts Britannica
Atoms form two basic bonds, covalent or ionic bonds, to fill the full outer shell of electrons. So those electrons belong to both of those atoms. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Because of its position midway in the second horizontal row of the periodic table,.
Biochemistry Honors
The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Ad generationgenius.com has been visited by 100k+ users in the past month Because of its position midway in the second horizontal row of the periodic table, carbon is neither an electropositive. Valence electrons are the electrons in the outer energy.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Because of its position midway in the second horizontal row of the periodic table, carbon is neither an electropositive. Interpret the properties of elements that are important for life from the periodic table. When a hydrogen atom loses its single electron. Web to achieve a full valence electrons shell. A covalent bond is formed when.
Ppt Atomic Structure And Chemical Bonding Powerpoint Presentation 7F5
Ad browse & discover thousands of science book titles, for less. Web main types of chemical bonds. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with other elements but also with itself. Web the atoms of molecules are linked together through a reaction known.
PPT Types of Bonds PowerPoint Presentation, free download ID1996079
Web bonds form when atoms share or transfer valence electrons. Web why do atoms form bonds with each other? Web we would like to show you a description here but the site won’t allow us. Web all models of chemical bonding have three common features: There are three different types of. Chemical bonds are formed.
PPT Chapter 7 Atoms & Bonding PowerPoint Presentation, free download
Atomic structure of carbon atom showing the particles of an atom: A helium atom (atomic number 2), has two protons and two electrons. The bond length is the internuclear distance at which the lowest. Web all models of chemical bonding have three common features: Valence electrons are the electrons in the outer energy level of.
What Happens When Atoms Bond infographic diagram showing how electrons
A covalent bond is formed when atoms share valence electrons. Atoms form bonds because the products are more stable than the isolated atoms; Ad browse & discover thousands of science book titles, for less. Understand why and how atoms form bonds. Interpret the properties of elements that are important for life from the periodic table..
Chemical Bonds · Anatomy and Physiology
Web bonds form when atoms share or transfer valence electrons. Web the atoms of molecules are linked together through a reaction known as chemical bonding. Atomic structure of carbon atom showing the particles of an atom: It becomes a positive hydrogen ion (h+). Ad generationgenius.com has been visited by 100k+ users in the past month.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. Classify the following bonds as ionic, polar covalent, or nonpolar covalent: Web the atoms of molecules are linked together through a reaction known as chemical bonding. Covalent bond is a chemical bond that involves.
Why Do Atoms Form Chemical Bonds With Other Atoms There are three different types of. Ad browse & discover thousands of science book titles, for less. Interpret the properties of elements that are important for life from the periodic table. Web to achieve a full valence electrons shell. Web the atoms of molecules are linked together through a reaction known as chemical bonding.
To Understand Why Atoms Form Molecules.
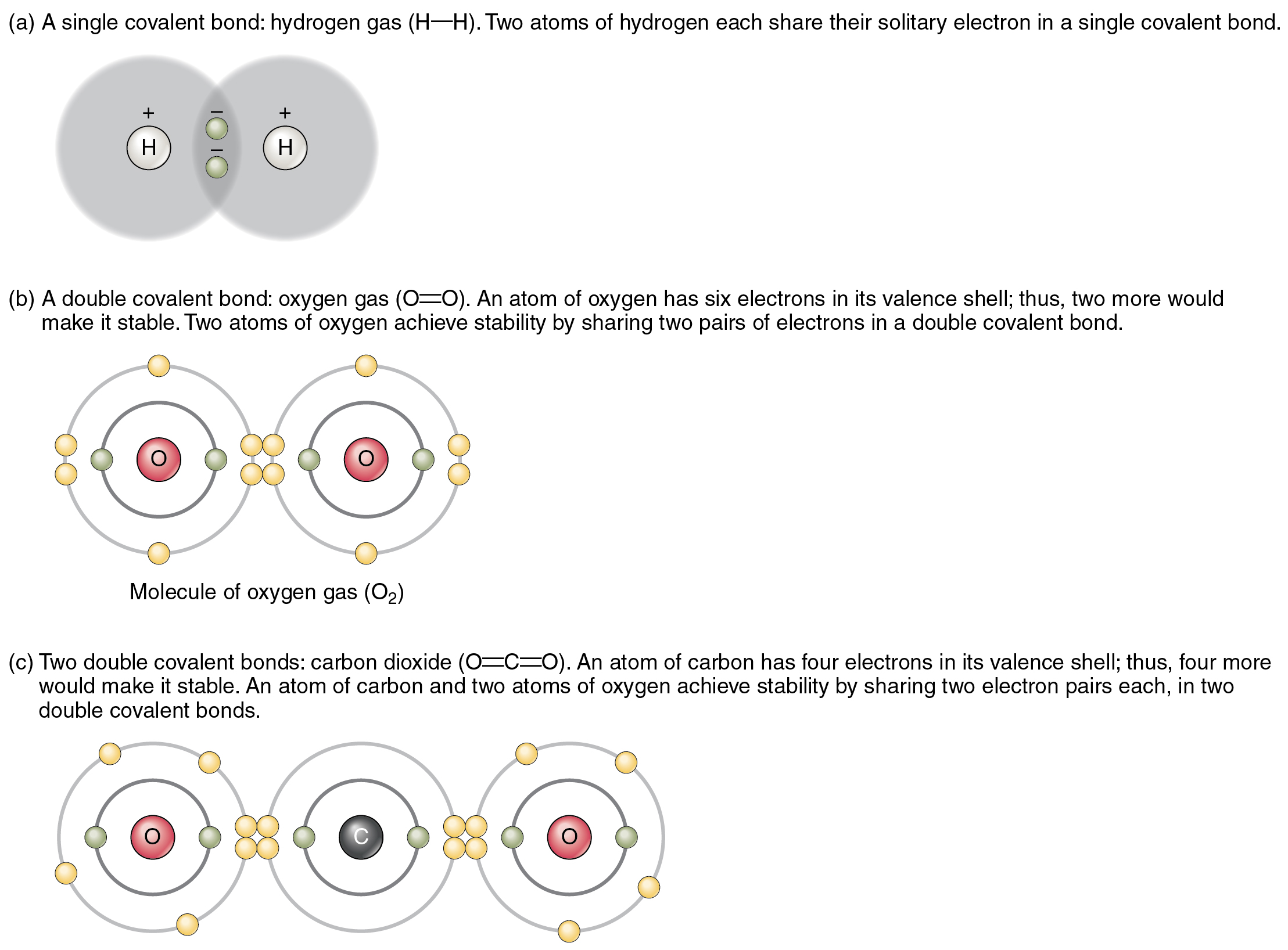
An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. Covalent bond is a chemical bond that involves sharing of electron pairs between atoms. Atoms form bonds because the products are more stable than the isolated atoms; A hydrogen atom (atomic number 1) has one proton and a lone electron, so it can readily share its electron with the outer shell of another atom.
Because Of Its Position Midway In The Second Horizontal Row Of The Periodic Table, Carbon Is Neither An Electropositive.
The very first electron shell only holds two electrons. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with other elements but also with itself. Bonding interactions are characterized by a particular energy (the bond energy or lattice energy), which is the amount of energy required to dissociate the substance into its components; Just as atoms can form molecular bonds with other atoms (especially in molecules), some molecules can form bonds with other molecules, as with (oh) radicals and hydrated molecules.
Atoms Form Chemical Bonds To Achieve A Full Outer Energy Level, Which Is The Most Stable Arrangement Of Electrons.
Atomic structure of carbon atom showing the particles of an atom: The potential energy of two separate hydrogen atoms (right) decreases as they approach each other, and the single electrons on each atom are shared to form a covalent bond. Bonds form when atoms share or transfer valence electrons. Web bonds form when atoms share or transfer valence electrons.
Web A Chemical Bond Is A Force Of Attraction Between Atoms Or Ions.
Web just as the structure of the atom is held together by the electrostatic attraction between the positively charged nucleus and the negatively charged electrons surrounding it, the stability within chemical bonds is also due to electrostatic attractions. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Valence electrons are the basis of all chemical bonds. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more stable than when the atoms are apart.