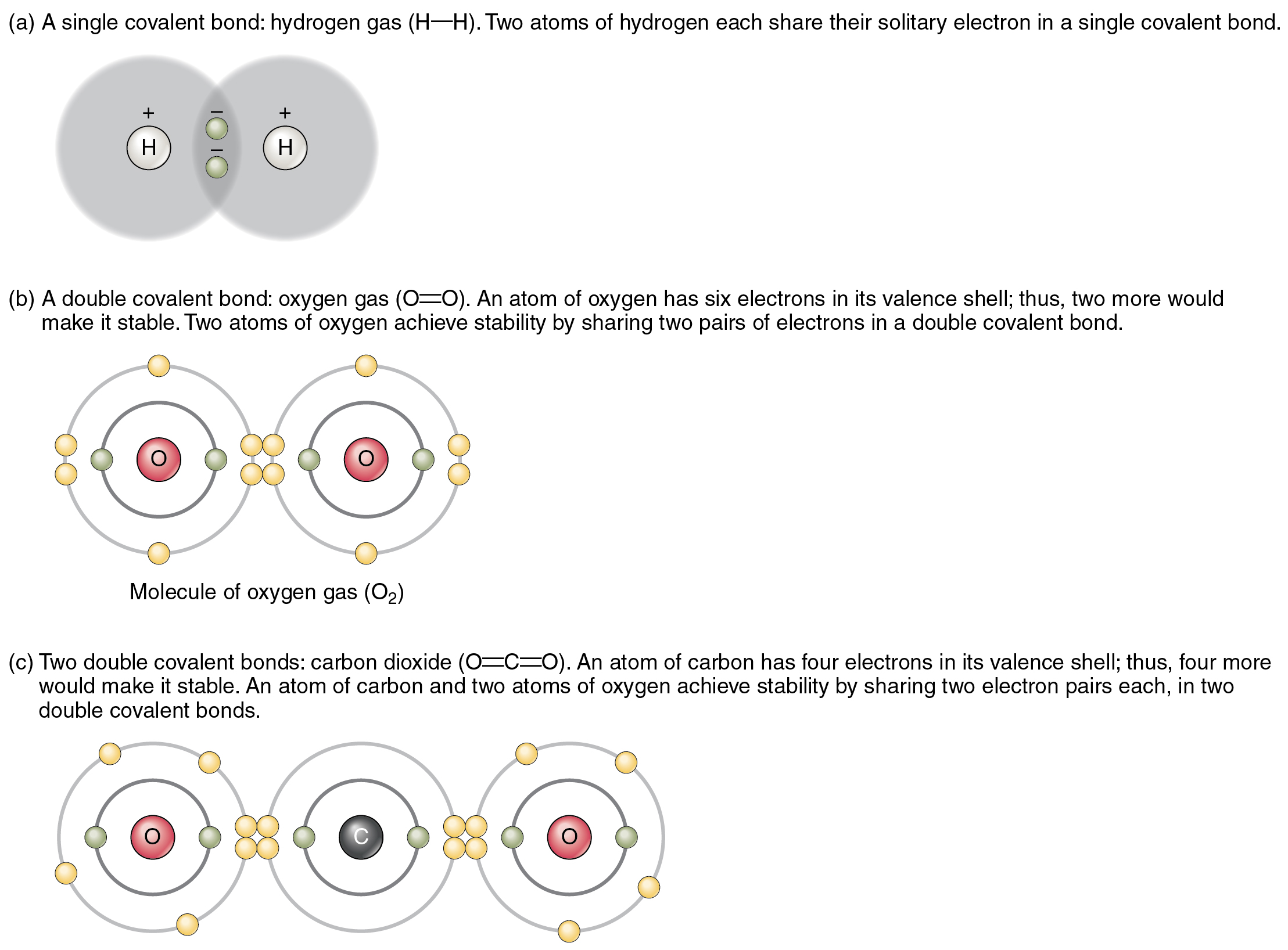
How Many Covalent Bonds Can Nitrogen Form - Answer verified 292.8k + views hint:
How Many Covalent Bonds Can Nitrogen Form - Web answer link nitrogen typically forms 3 covalent bonds, including in n_2. A single covalent bond b. Web how can you tell the number covalent bonds the atoms of an element can form? One lone pair and three unpaired electrons. Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol:
To obtain an octet, these atoms. To obtain an octet, these atoms. Is determined by the distance at which the lowest potential energy is achieved. Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: Web group 5a elements such as nitrogen have five valence electrons in the atomic lewis symbol: Web the reason that ncl 5 doesn't exist is that in order to form five bonds, the nitrogen would have to promote one of its 2s electrons. A single covalent bond b.
How are elements and atoms related? + Example
One lone pair and three unpaired electrons. Thus, bonding in potassium nitrate is ionic, resulting from the electrostatic. A double covalent bond c. The problem is that there aren't. Web nitrogen has 7 electrons which are configured as (2, 5). One lone pair and three unpaired electrons. So when nitrogen has two bonds and. The.
Q&As How much N is Left? Do I Need More Starter When Replanting
Web group 5a elements such as nitrogen have five valence electrons in the atomic lewis symbol: Web atoms of different elements close element a substance made of one type of atom only. Group 5a (15) elements such as nitrogen. A triple covalent bond d. Web answer link nitrogen typically forms 3 covalent bonds, including in.
What is Nitrogen? Definition, Formula, Cycle, Fixation
A triple covalent bond d. Web atoms of different elements close element a substance made of one type of atom only. Answer verified 292.8k + views hint: For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two. Web answer link nitrogen typically forms 3 covalent bonds, including in n_2. Web.
The Covalent Bond CK12 Foundation
How many covalent bonds can nitrogen form? To obtain an octet, these atoms. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ch 4 (methane). Web cbse notes live join vedantu’s free mastercalss how many single covalent bonds can nitrogen form? The potential energy of two.
Bond Formation In Nitrogen Molecule Photograph by
A single covalent bond b. Web how can you tell the number covalent bonds the atoms of an element can form? To obtain an octet, these atoms. Answer verified 292.8k + views hint: This means that hydrogen needs three electrons in order to saturate its outermost energy level and. So when nitrogen has two bonds.
Chemical Bonds · Anatomy and Physiology
Will form either one, two, three or four covalent bonds with other atoms. Web cbse notes live join vedantu’s free mastercalss how many single covalent bonds can nitrogen form? Is determined by the distance at which the lowest potential energy is achieved. Web group 5a elements such as nitrogen have five valence electrons in the.
Nitrogen Definition, Symbol, Uses, Properties, Atomic Number, & Facts
To obtain an octet, these atoms. A triple covalent bond d. The carbon atom is unique among elements in its tendency to form extensive networks of covalent. Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: A double covalent bond c. Web the pattern for a formal.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web nitrogen has 7 electrons which are configured as (2, 5). Web atoms of different elements close element a substance made of one type of atom only. One lone pair and three unpaired electrons. Is determined by the distance at which the lowest potential energy is achieved. How many covalent bonds can nitrogen form? One.
Covalent Bonds Biology for NonMajors I
Web two different atoms can also share electrons and form covalent bonds. A triple covalent bond d. Web cbse notes live join vedantu’s free mastercalss how many single covalent bonds can nitrogen form? To obtain an octet, these atoms. This is because it has atomic number 7, so its electron configuration is 1s^2 2s^2 2p^3,..
Covalent bonding in an oxygen molecule. Chemistry Activities, Gcse
One lone pair and three unpaired electrons. The carbon atom is unique among elements in its tendency to form extensive networks of covalent. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two. A double covalent bond c. Web for example, potassium nitrate, kno 3, contains the k + cation.
How Many Covalent Bonds Can Nitrogen Form Web cbse notes live join vedantu’s free mastercalss how many single covalent bonds can nitrogen form? Is determined by the distance at which the lowest potential energy is achieved. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two. Will form either one, two, three or four covalent bonds with other atoms. Web group 5a elements such as nitrogen have five valence electrons in the atomic lewis symbol:
A Single Covalent Bond B.
The potential energy of two separate hydrogen atoms (right) decreases as. Web group 5a elements such as nitrogen have five valence electrons in the atomic lewis symbol: Web the reason that ncl 5 doesn't exist is that in order to form five bonds, the nitrogen would have to promote one of its 2s electrons. Web atoms of different elements close element a substance made of one type of atom only.
So When Nitrogen Has Two Bonds And.
One lone pair and three unpaired electrons. Web nitrogen has 7 electrons which are configured as (2, 5). To obtain an octet, these atoms. Is determined by the distance at which the lowest potential energy is achieved.
This Is Because It Has Atomic Number 7, So Its Electron Configuration Is 1S^2 2S^2 2P^3,.
Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion. Web two different atoms can also share electrons and form covalent bonds. Web the atoms in the nitrogen molecule, n2, are held together by: How many covalent bonds can nitrogen form?
For Example, Water, (\(\Ce{H2O}\)), Has Two Covalent Bonds Between A Single Oxygen Atom And Two.
Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: Will form either one, two, three or four covalent bonds with other atoms. One lone pair and three unpaired electrons. Web how can you tell the number covalent bonds the atoms of an element can form?