Draw The Electron Configuration For A Neutral Atom Of Aluminum. - The next six electrons can go in the 2p orbital.
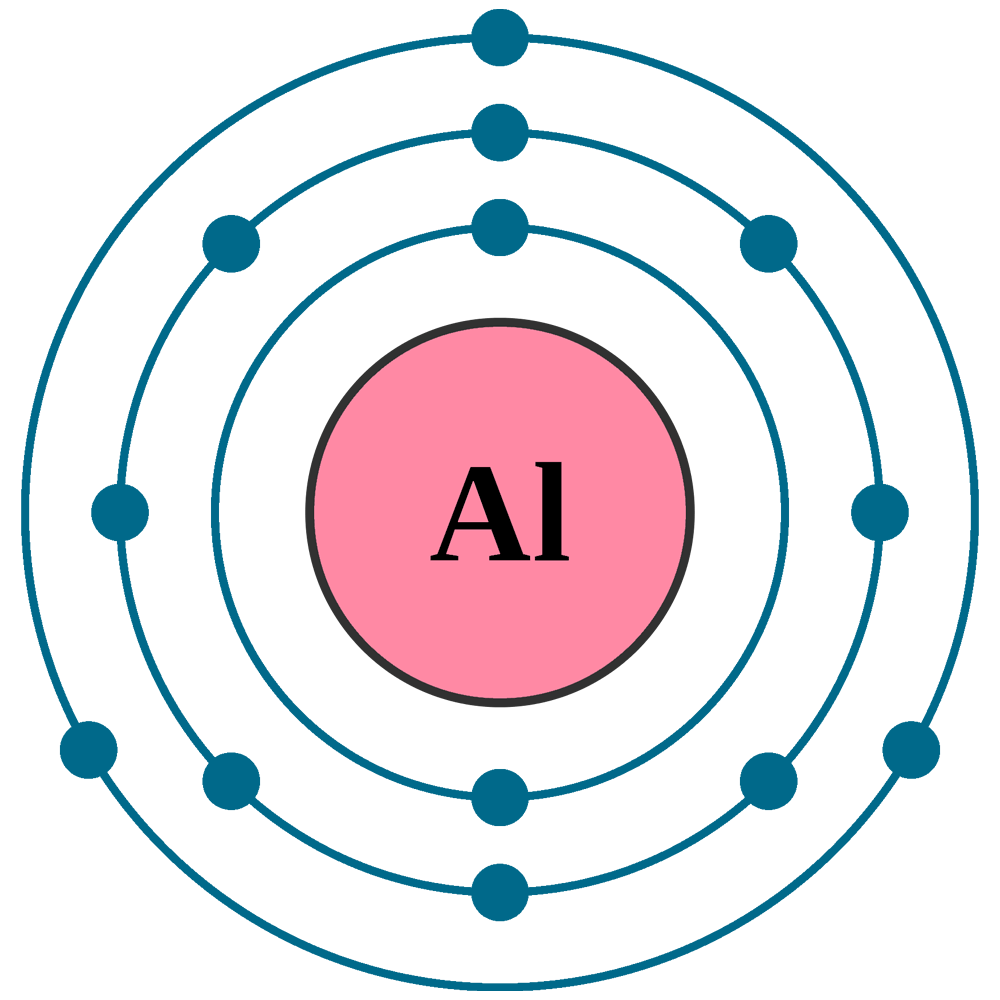
Draw The Electron Configuration For A Neutral Atom Of Aluminum. - The first two electrons can go in the 1s orbital. Aluminium is located in period 3, group 13, and has an atomic number equal to #13#. Web the first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1 s2 2 s2 2 p6 3 s2 3 p1. The electron configuration of aluminum is 1s^2 2s^2 2p^6 3s^2 3p^1 to draw the electron configuration put al in the middle of a circle place two dots next to the circle and draw a second circle leaving only the two dots. The next two electrons can go in the 2s orbital.
This tells you that the electron configuration of a neutral aluminium atom must account for a total of #13# electrons. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This problem has been solved! \[\dot{al:} \nonumber \nonumber \] the valence electron configuration for selenium is 4s 2 4p 4. The next two electrons can go in the 2s orbital. This problem has been solved!
SOLVED Draw the electron configuration for & neutral atom of aluminum
Web an electrically neutral atom has the following electron configuration: Aluminum is the 13th element on the periodic table, so it has 13 electrons in a neutral state. This problem has been solved! \[\dot{al:} \nonumber \nonumber \] the valence electron configuration for selenium is 4s 2 4p 4. Web the arrangement of electrons in aluminum.
How Can We Find Electron Configuration For Aluminium (Al)
Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Aluminum number 13 has 13 dots around it. 1s 3p 2p 2s 3s this problem has been solved! An aluminium atom has 13 electrons, arranged in an electron.
Diagram representation of the element aluminium Vector Image
This video discusses how to write electron configurations for h, li, c and sc. The next two electrons can go in the 2s orbital. Aluminium is located in period 3, group 13, and has an atomic number equal to #13#. Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely.
Aluminium electronic configuration How to Write Aluminium electronic
Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Web electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of sulfur. An aluminium atom has.
Bohr Model Electron Aluminium Lewis Structure Atom, PNG, 600x590px
This tells you that the electron configuration of a neutral aluminium atom must account for a total of #13# electrons. Web these three dots represent the valance or bonding electrons of al. Web configuration of an atom draw the electron configuration for a neutral atom of aluminum. Electrons are represented by dots or crosses and.
Atomic structure of aluminum Brainly.in
Web electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of sulfur. Web faq this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. 1s 3p 2p 2s 3s this problem has.
Atom Diagrams Electron Configurations of the Elements
As per the aufbau rule, the electrons will be filled into 1s orbital first then 2s, then 2p…so on. The atomic number of al is 13. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. 1s 3p 2p 2s 3s this problem has been solved! Web the easiest way to create electron configurations is using an electron configuration.
Electron arrangements
1s 2 2s 2 2p 6 3s 1: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Energy this problem has been solved! 1s 2 2s 2 2p 6: Web step 1/6 1. Web draw the electron configuration for a neutral atom of aluminum. Web in order to.
How Can We Find Electron Configuration For AL (Aluminium)
When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom. We'll represent this as 1s². Aluminium is located in period 3, group 13, and has an atomic number equal to #13#. Web the valence electron configuration for aluminum is 3s 2 3p 1. What is the.
Aluminium Al (Element 13) of Periodic Table Elements FlashCards
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 1s 3p 2p 2s 3s this problem has been solved! Web configuration of an atom draw the electron configuration for a neutral atom of aluminum. Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to.
Draw The Electron Configuration For A Neutral Atom Of Aluminum. Aluminum is the 13th element on the periodic table, so it has 13 electrons in a neutral state. This video discusses how to write electron configurations for h, li, c and sc. It looks something like this. Aluminium is located in period 3, group 13, and has an atomic number equal to #13#. We'll represent this as 1s².
Electrons Are Represented By Dots Or Crosses And Are Positioned In Energy Levels, Or ‘Shells’, Around The Central Nucleus.
Web the electron configuration for aluminum is: An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. The electron configuration of aluminum is [ ne] 3s 2 3p 1 , if the electron arrangement is through orbitals. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
However, a curious thing happens after the 3 p subshell is filled: Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. 1s 2 2s 2 2p 5: The next two electrons can go in the 2s orbital.
As Per The Aufbau Rule, The Electrons Will Be Filled Into 1S Orbital First Then 2S, Then 2P…So On.
An atom has a valence shell electron configuration of #ns^1#. Aluminum number 13 has 13 dots around it. We'll represent this as 1s². Web an aluminum atom is a neutral atom that has an atomic number of 13 which implies it has a total of 13 electrons.
The Atomic Number Of Al Is 13.
Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Web electron configuration of oxygen (o) [he] 2s 2 2p 4: 1s 2 2s 2 2p 6 3s 1:







:max_bytes(150000):strip_icc()/aluminiumatom-58b602655f9b5860464c6f7d.jpg)