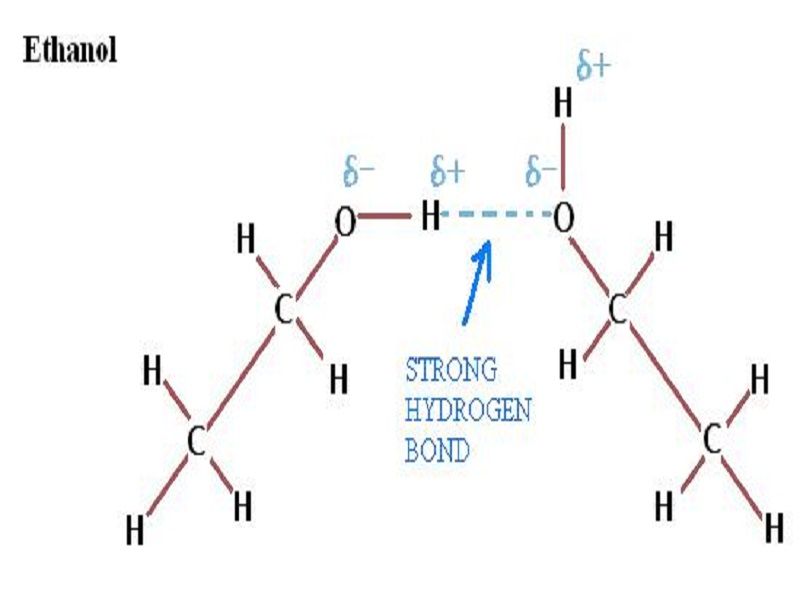
Can Ethanol Form Hydrogen Bonds - Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water.
Can Ethanol Form Hydrogen Bonds - (b) structures of the dimethyl ether molecule and the ethanol molecule are. Web the answer is usually a metal. Web we would like to show you a description here but the site won’t allow us. The strongly electronegative elements (primarily nitrogen, oxygen, and fluorine) will always form a relatively large. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons.
This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. In order to mix the two, the hydrogen bonds between water molecules and the hydrogen bonds between ethanol molecules must be broken. Web in summary, ethanol molecules can form hydrogen bonds through the oxygen atom's two lone pairs and the hydrogen atom's ability to donate a hydrogen. Web the answer is usually a metal. At least at first glance, it's not an element that suggests metallic properties. (b) structures of the dimethyl ether molecule and the ethanol molecule are. Web we would like to show you a description here but the site won’t allow us.
Does Ethanol Form Hydrogen Bonds Printable Form, Templates and Letter
Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. As a result, alcohols have boiling points that are much higher than alkanes with similar molecular. Then, there's hydrogen, a colorless and odorless gas. The strongly electronegative elements (primarily nitrogen, oxygen, and fluorine) will always form a relatively large. At.
PPT Chapter 4 Alcohols and Alkyl Halides PowerPoint Presentation
The strongly electronegative elements (primarily nitrogen, oxygen, and fluorine) will always form a relatively large. Web can butane, acetone, and ethanol form a hydrogen bond with: As a result, alcohols have boiling points that are much higher than alkanes with similar molecular. The hydrogen bonding is limited by the fact that there is only one..
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates
Web the answer is usually a metal. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web to understand hydrogen bonding in ethanol (c2h5oh) we need to know that ethanol is a polar molecule. Web hydrogen bonds cannot form between ester molecules, but they.
Physical Properties of Alcohols Easy exam revision notes for GSCE
Web to understand hydrogen bonding in ethanol (c2h5oh) we need to know that ethanol is a polar molecule. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web hydrogen bonds cannot form between ester molecules, but they can form between alcohol and carboxylic acid.
Does Ethanol Form Hydrogen Bonds Printable Form, Templates and Letter
The strongly electronegative elements (primarily nitrogen, oxygen, and fluorine) will always form a relatively large. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. In order to mix the two, the hydrogen bonds between water molecules and the hydrogen bonds between ethanol molecules must.
9 Hydrogen Bond Examples in Real Life StudiousGuy
Web the answer is usually a metal. Web hydrogen bonds cannot form between ester molecules, but they can form between alcohol and carboxylic acid molecules. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web alcohols can form similar hydrogen bonds, as shown in the figure below. This is.
PPT Chapter 4 Alcohols and Alkyl Halides PowerPoint Presentation
This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web we would like to show you a description here but the site won’t allow us. Web the answer is usually a metal. Web to understand hydrogen bonding in ethanol (c2h5oh) we need.
Alcohols (ALevel) ChemistryStudent
(b) structures of the dimethyl ether molecule and the ethanol molecule are. In order to mix the two, the hydrogen bonds between water molecules and the hydrogen bonds between ethanol molecules must be broken. This means that it has a positive and a negative side. Web we would like to show you a description here.
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates
This means that it has a positive and a negative side. (b) structures of the dimethyl ether molecule and the ethanol molecule are. Web the answer is usually a metal. As a result, alcohols have boiling points that are much higher than alkanes with similar molecular. Web a water molecule consists of two hydrogen atoms.
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates
At least at first glance, it's not an element that suggests metallic properties. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web alcohols can form similar hydrogen bonds, as shown in the figure below. Web to understand hydrogen bonding in ethanol (c2h5oh) we need to know that ethanol.
Can Ethanol Form Hydrogen Bonds This means that it has a positive and a negative side. Web in both pure water and pure ethanol the main intermolecular attractions are hydrogen bonds. Web can butane, acetone, and ethanol form a hydrogen bond with: As a result, alcohols have boiling points that are much higher than alkanes with similar molecular. Web to understand hydrogen bonding in ethanol (c2h5oh) we need to know that ethanol is a polar molecule.
Web Can Butane, Acetone, And Ethanol Form A Hydrogen Bond With:
This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. In order to mix the two, the hydrogen bonds between water molecules and the hydrogen bonds between ethanol molecules must be broken. Web we would like to show you a description here but the site won’t allow us. At least at first glance, it's not an element that suggests metallic properties.
The Strongly Electronegative Elements (Primarily Nitrogen, Oxygen, And Fluorine) Will Always Form A Relatively Large.
Web the answer is usually a metal. This means that it has a positive and a negative side. Web to understand hydrogen bonding in ethanol (c2h5oh) we need to know that ethanol is a polar molecule. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water.
Web Alcohols Can Form Similar Hydrogen Bonds, As Shown In The Figure Below.
Web hydrogen bonds cannot form between ester molecules, but they can form between alcohol and carboxylic acid molecules. Web in both pure water and pure ethanol the main intermolecular attractions are hydrogen bonds. Web in summary, ethanol molecules can form hydrogen bonds through the oxygen atom's two lone pairs and the hydrogen atom's ability to donate a hydrogen. Then, there's hydrogen, a colorless and odorless gas.
Show Where The Hydrogen Bond Forms In A Lewis Structure.
As a result, alcohols have boiling points that are much higher than alkanes with similar molecular. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. This is why methyl methanoate has a lower. (b) structures of the dimethyl ether molecule and the ethanol molecule are.