Amino Acids That Can Form Hydrogen Bonds - You may know that it requires three things:
Amino Acids That Can Form Hydrogen Bonds - The method could be used not only for synthesizing amides from carboxylic. (figure 1) draw it as it would. Web the hydrogen bonds form between the oxygen atom in the carbonyl group in one amino acid and another amino acid that is four amino acids farther along the chain. The two amino acids in this group are aspartic acid and glutamic acid. Tertiary structure is stabilized by a combination of forces, including.
Molecule which bears charged groups of opposite polarity. Web 1 day agoscience biochemistry proteins are made from chains of amino acids. A hydrogen atom (check) bonded to an electronegative atom (typically $\ce{o, n, f}$ — check) and another electronegative atom that can receive (typically the same atoms — check) so a hydrogen bond is possible where an ionic interaction is not. The method could be used not only for synthesizing amides from carboxylic. Web the polar, uncharged amino acids serine (ser, s), threonine (thr, t), asparagine (asn, n) and glutamine (gln, q) readily form hydrogen bonds with water and other amino acids. You may know that it requires three things: A) arginine and glutamic acid.
(a) Hydrogen bonding patterns that describe the αand πconfigurations
Web the carbonyl group can function as a hydrogen bond acceptor, and the amino group (nh 2) can function as a hydrogen bond donor. Molecule which bears charged groups of opposite polarity. Ion pairing is one of the most important noncovalent forces in chemistry, in. Web polar amino acids (form hydrogen bonds as proton donors.
Amino acids physical, chemical properties and peptide bond
For this problem, draw all hydrogen atoms explicitly. Web a hydrogen bond is a very viable possibility, though. Web amino acids with polar groups that form hydrogen bonds to water are classified as. Hydrogen bonds can exist between atoms in different molecules or in the same molecule. This is an example of severe perturbation, and.
PPT Introduction to Amino Acids of Medical Importance PowerPoint
Web polar amino acids (form hydrogen bonds as proton donors or acceptors): The weak bonds are of three types: The forces in secondary structure primarily involve hydrogen bonds. Web the hydrogen bonds form between the oxygen atom in the carbonyl group in one amino acid and another amino acid that is four amino acids farther.
Two amino acids are joined together by
The two amino acids in this group are aspartic acid and glutamic acid. Web the essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. Web because the polar side chains of these amino acids can form hydrogen bonds with water, these amino acids are hydrophilic and tend to be located.
PPT Proteins PowerPoint Presentation, free download ID1828850
Molecule which bears charged groups of opposite polarity. The method could be used not only for synthesizing amides from carboxylic. Ion pairing is one of the most important noncovalent forces in chemistry, in. Hydrogen bonds can exist between atoms in different molecules or in the same molecule. Web hydrogen bonding, interaction involving a hydrogen atom.
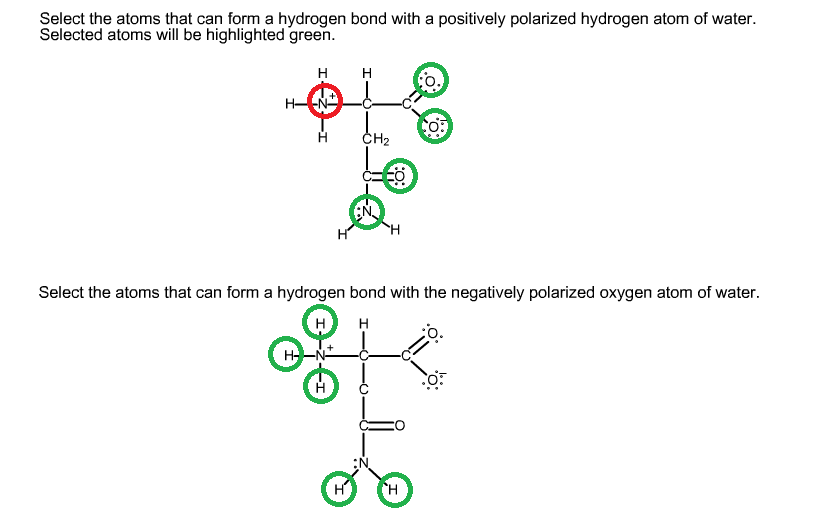
organic chemistry Which atoms in a given amino acid are able to form
Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving the two… hydrogen bonds. Which amino acids are involved in turns and kinks? Hydrophobic.
amino acids salt bridge vs hydrogen bond Chemistry Stack Exchange
Please explain why that is the correct answer. There are two requirements for hydrogen bonding. Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and.
Solved 18. The side chain of which amino acid can form
These involve atoms in the polypeptide backbone, as well as atoms in the amino acid side chains. A few biologically important derivatives of the standard amino acids are shown in the figure below. Molecule which bears charged groups of opposite polarity. Web polar amino acids (form hydrogen bonds as proton donors or acceptors): Each turn.
Chapter 22 Presentation
The amino acids lysine, arginine, and histidine have side chains with charged basic groups. Each turn of the helix spans 3.6 amino acids. Hydrophobic side chains interact with each other via weak van der waals. Web polar amino acids (form hydrogen bonds as proton donors or acceptors): The method could be used not only for.
PPT Inner Life of a Cell PowerPoint Presentation, free download ID
Each turn of the helix spans 3.6 amino acids. Part a draw the dipeptide that results when a peptide bond is formed between the two glycine molecules shown here. Amino acids are a crucial, yet basic unit of protein, and they contain an amino group and a carboxylic group. Web the hydrogen bonds form between.
Amino Acids That Can Form Hydrogen Bonds The weak bonds are of three types: Web charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. There are two requirements for hydrogen bonding. Hydrogen bonds can exist between atoms in different molecules or in the same molecule. You may know that it requires three things:
What Amino Acid Participated In Disulfide Bonds?
The weak bonds are of three types: The two amino acids in this group are aspartic acid and glutamic acid. The method could be used not only for synthesizing amides from carboxylic. Please explain why that is the correct answer.
A Few Biologically Important Derivatives Of The Standard Amino Acids Are Shown In The Figure Below.
These form hydrogen bonds to a purine, pyrimidine, or phosphate group in dna. Web the polar, uncharged amino acids serine (ser, s), threonine (thr, t), asparagine (asn, n) and glutamine (gln, q) readily form hydrogen bonds with water and other amino acids. There are two requirements for hydrogen bonding. Amino acids can be linked by a condensation reaction in which an ―oh is lost from the carboxyl group of one amino acid along with a hydrogen from the amino group of a second, forming a molecule of water and leaving the two… hydrogen bonds.
Hydrogen Bonds Can Exist Between Atoms In Different Molecules Or In The Same Molecule.
Web because the polar side chains of these amino acids can form hydrogen bonds with water, these amino acids are hydrophilic and tend to be located on the outside of proteins. Web a hydrogen bond is a very viable possibility, though. Web which amino acids can form hydrogen bonds? They play an extensive role in gene expression process, which includes an adjustment of protein functions that facilitate messenger rna (mrna) translation (scot et al., 2006).
Hydrogen Bonding And Ionic Bonding (Figure 1).
Which amino acids are involved in turns and kinks? Web which pair of amino acids can form hydrogen bonds between their r groups? (figure 1) draw it as it would. Web charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds.