A Carbon Atom Is Most Likely To Form - Web a carbon atom is most likely to form what kind of bond (s) with other atoms?
A Carbon Atom Is Most Likely To Form - A carbon atom is most likely to form what kind of bond (s) with other atoms? Web a carbon atom is most likely to form what kind of bond (s) with other atoms? Web because of its position midway in the second horizontal row of the periodic table, carbon is neither an electropositive nor an electronegative element; A carbon atom is most likely to form covalent bond (s) with other atoms. So, the correct answer is:
This problem has been solved! Web study with quizlet and memorize flashcards containing terms like which element is most likely to make two covalent bonds in the formation of a molecular compound?, which. A) ionic bonds b) hydrogen bonds c) covalent bonds d) covalent bonds and. Web a carbon atom is most likely to form which of the following bonds with other atoms? Which atom is most likely to form a polar covalent bond with carbon? Covalent what determines whether a carbon atom's covalent bonds to other atoms are in. Carbon atom is a group 14 element and its atomic number is 6.
How do the bonding properties of carbon atoms allow for the large
Web a carbon atom is most likely to form what kind of bond(s) with other atoms? This is because a carbon atom has four valence electrons and will form bonds… So, the correct answer is: It therefore is more likely to. How many bonds will carbon commonly form? How many bonds will oxygen commonly form?..
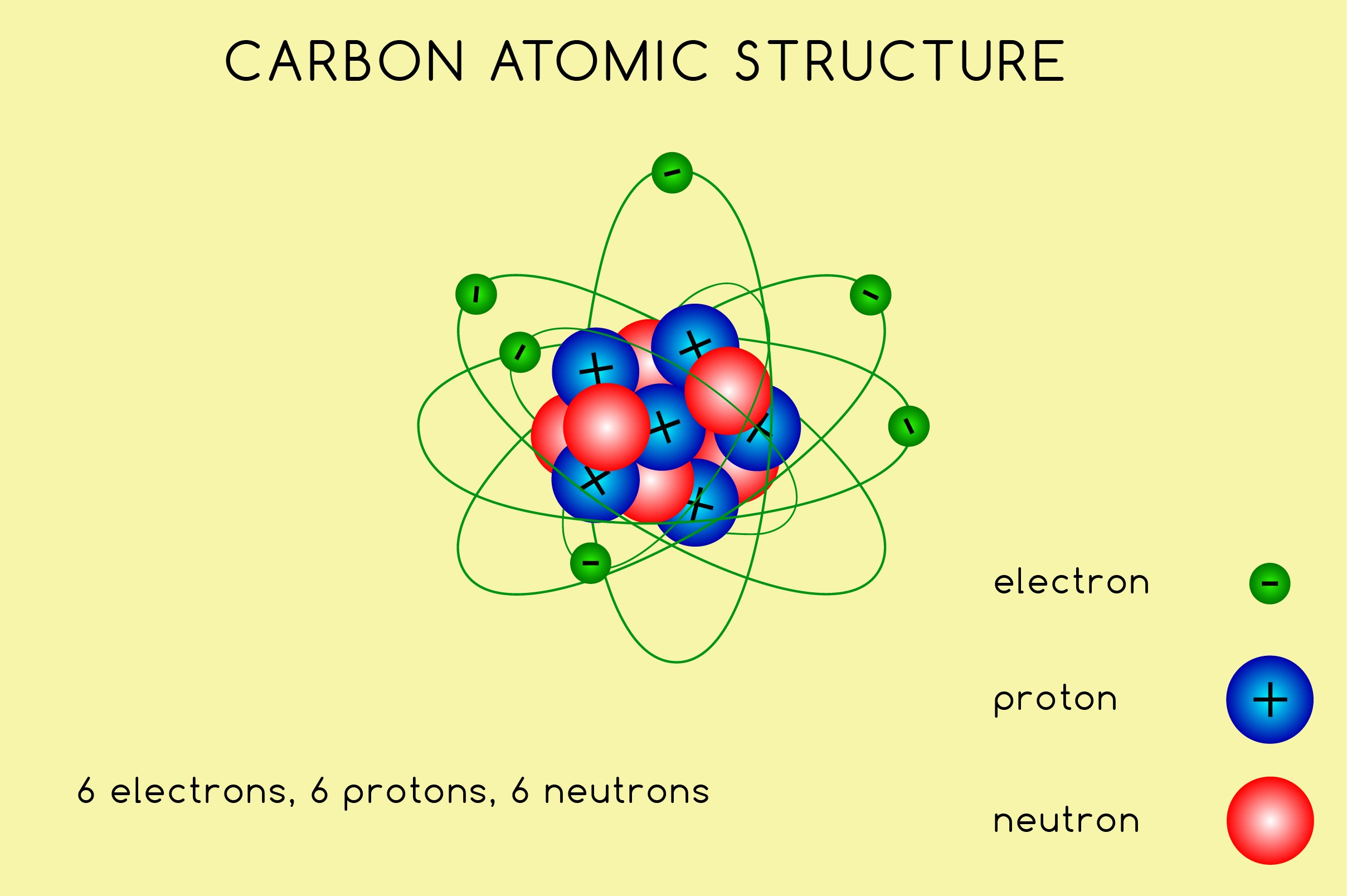
Carbon atom diagram concept Royalty Free Vector Image
Covalent what determines whether a carbon atom's covalent bonds to other atoms are in. Which atom is most likely to form a polar covalent bond with carbon? O ionic o hydrogen covalent o ionic bonds, covalent bonds, and hydrogen. Web in cho 2 −, cho 2 −, the less electronegative carbon atom occupies the central.
PPT CARBON PowerPoint Presentation, free download ID3108318
Web a carbon atom is most likely to form what kind of bond (s) with other atoms? This is because a carbon atom has four valence electrons and will form bonds… Carbon can form a variety of bonds in nature. Web in cho 2 −, cho 2 −, the less electronegative carbon atom occupies the.
Carbon atomic structure (437243) Illustrations Design Bundles
Web carbon is the most electronegative element in the periodic table. Choose the term that correctly describes the relationship between these two sugar. Web a carbon atom is most likely to form what kind of bond(s) with other atoms? How many bonds will carbon commonly form? O ionic o hydrogen covalent o ionic bonds, covalent.
Carbon Facts about an element that is a key ingredient for life on
Web a carbon atom is most likely to form what kind of bond(s) with other atoms? Web carbon is the most electronegative element in the periodic table. So, the correct answer is: Web a carbon atom is most likely to form covalent bonds with other atoms. Carbon atom is a group 14 element and its.
Carbon Atom Ascension Glossary
Web broadly speaking, all pfas have a chain of carbon atoms bonded to fluorine atoms. Other examples include p in pocl. How many bonds will nitrogen commonly form? How many electrons must one carbon atom. So, the correct answer is: The most common bond formed by carbon is a covalent bond. Web a carbon atom.
Carbon — Role and Importance to Life Expii
Carbon atom is a group 14 element and its atomic number is 6. So, the correct answer is: Other examples include p in pocl. How many bonds will oxygen commonly form?. Web a carbon atom is most likely to form which of the following bonds with other atoms? Choose the term that correctly describes the.
A Carbon Atom Is Most Likely To Form What Kind Of Bond(S) With Other
Choose the term that correctly describes the relationship between these two sugar. Web broadly speaking, all pfas have a chain of carbon atoms bonded to fluorine atoms. Web in cho 2 −, cho 2 −, the less electronegative carbon atom occupies the central position with the oxygen and hydrogen atoms surrounding it. How many bonds.
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
This is because a carbon atom has four valence electrons and will form bonds… Web a carbon atom is most likely to form what kind of bond (s) with other atoms? Web study with quizlet and memorize flashcards containing terms like which element is most likely to make two covalent bonds in the formation of.
A Carbon Atom Is Most Likely To Form What Kind Of Bond(S) With Other
Carbon can form a variety of bonds in nature. How many bonds will carbon commonly form? It therefore is more likely to. This problem has been solved! Web a carbon atom is most likely to form covalent bonds with other atoms. A carbon atom is most likely to form covalent bond (s) with other atoms..
A Carbon Atom Is Most Likely To Form Which atom is most likely to form a polar covalent bond with carbon? So, the correct answer is: O ionic o hydrogen covalent o ionic bonds, covalent bonds, and hydrogen. Web a carbon atom is most likely to form a covalent bond with other others. This problem has been solved!
Ad Browse & Discover Thousands Of Science Book Titles, For Less.
Web a carbon atom is most likely to form what kind of bond (s) with other atoms? How many electrons must one carbon atom. This is because a carbon atom has four valence electrons and will form bonds… It therefore is more likely to.
A Carbon Atom Is Most Likely To Form Covalent Bond (S) With Other Atoms.
Web a carbon atom is most likely to form what kind of bond(s) with other atoms? Web study with quizlet and memorize flashcards containing terms like which element is most likely to make two covalent bonds in the formation of a molecular compound?, which. Web a carbon atom is most likely to form which of the following bonds with other atoms? A carbon atom is most likely to form what kind of bond (s) with other atoms?
Web A Carbon Atom Is Most Likely To Form What Kind Of Bond(S) With Other Atoms?
How many bonds will oxygen commonly form?. Web because of its position midway in the second horizontal row of the periodic table, carbon is neither an electropositive nor an electronegative element; A) ionic bonds b) hydrogen bonds c) covalent bonds d) covalent bonds and. Other examples include p in pocl.
Web In Cho 2 −, Cho 2 −, The Less Electronegative Carbon Atom Occupies The Central Position With The Oxygen And Hydrogen Atoms Surrounding It.
Web a carbon atom is most likely to form a covalent bond with other others. How many bonds will carbon commonly form? Carbon can form a variety of bonds in nature. Which atom is most likely to form a polar covalent bond with carbon?